Reactive Metal Rings
FAU researchers build rings of cations and metallic magnesium
As a reminder: A few years ago a team around Professor Sjoerd Harder reported a breakthrough in the chemistry of magnesium (Nature 2021, 592, 717). Magnesium (Mg), which in chemical compounds is always charged 2+, was stabilized in the zero oxidation state and found to react as a nucleophile instead of an electrophile. Now borders in magnesium chemistry are moved again.
Redox-active inverse crown complexes:
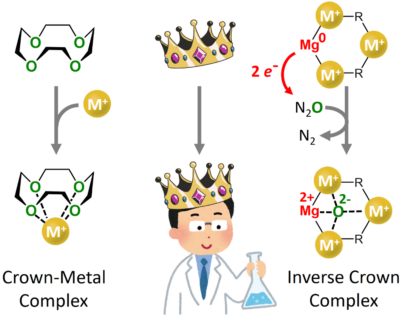
Cyclic ether molecules are able to selectively bind positively charged metal cations (M+). As such complexes resemble a crown, they are referred to as crown ether complexes. The enormous significance of such crown metal complexes led in 1987 to the Nobel prize for Cram, Lehn and Pedersen.
As one may have guessed, the building principle of inverse crown complexes is inverted: a ring of metal cations (M+) is able to selectively bind negatively charged anions. Now the team around Professor Sjoerd Harder, chair of inorganic and organometallic chemistry at the FAU, managed to integrate metallic magnesium in such inverse crowns. The new complex combines two important features. (1) Magnesium in the zero oxidation state is able to deliver two electrons and the complex is therefore strongly reducing. (2) The resulting negatively charged anions are then captured in the ring of positively charged cations. As the product is unusually stable, such reactions are extremely fast.
In their publication in the renowned journal Nature Chemistry the team shows for example fast decomposition of dinitrogen oxide (N2O), better known as laughing gas. Laughing gas is sedating and is used as an anaesthetic in dentistry or given during childbirth. It occurs naturally in the earth’s atmosphere as a trace gas and, like carbon dioxide (CO2), it is one of the greenhouse gases. However, it is nearly 300 times more efficient in global warming. Sources of N2O in the earth’s atmosphere are industry and intensive farming using nitrogen-containing fertilizers. As there is hardly any breakdown of N2O in the troposphere, it is an exceptionally long-living greenhouse gas. However, the highly reactive inverse crown complexes of the FAU team react within seconds with N2O, even at extremely low temperatures of –80 °C!
Although such high reactivity is a striking demonstration of its effectiveness as a reducing agent, the FAU team especially focused on the generation and capture of hitherto never isolated anions such as CO22ˉ. There is not a lot known about the carbonite anion but it is generally accepted that it plays a role in the electrochemical breakdown of CO2. As fundamental understanding of electron-transfer processes and intermediates are key to further advancing this chemistry, model complexes like the redox-active inverse crown are highly relevant.
Further Information
Original publication:
J. Maurer, L. Klerner, J. Mai, H. Stecher, S. Thum, M. Morasch, J. Langer, S. Harder Nature Chemistry 2025, https://www.nature.com/articles/s41557-024-01724-5
Contact:
Prof. Dr. Sjoerd Harder, PhD
Department of Chemistry and Pharmacy
Chair of Inorganic and Organometallic Chemistry (Prof. Dr. Harder)
- Phone number: +49913185-27350
- Email: sjoerd.harder@fau.de