Phase I – Achievements
Phase I – Achievements
During the first funding period, the joint effort and the interdisciplinary interaction between chemists, physicists, materials scientists, chemical engineers and theoreticians involved has proven to be a highly productive and synergistic basis for accomplishing breakthroughs in the field of carbon allotrope research. The overview over some outstanding achievements below documents the effectivity of the broad range of disciplines networking and collaborating at the SFB 953 and shows the pioneering qualities that makes this research program one of the foremost entities of carbon allotrope research in the world.
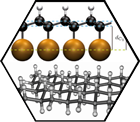 Both wet-chemical and surface supported approaches have been followed. The highest degree of bulk hydrogenation was obtained in a Birch-type reaction, where frozen water (ice) was used as a gentle proton source. We were able to show, for the first time, that indeed wet chemistry can be used to efficiently generate poly-hydrogenated graphene, which is a milestone achievement on the way to graphane. The reaction product was characterized by statistical Raman spectroscopy and microscopy (SRS, SRM), by IR-spectroscopy, optical spectroscopy (absorption and emission), TGA/MS, and high resolution TEM. For comparison deuterated graphene was also prepared using the same reaction conditions allowing for the unambiguous detection of the expected C-D-vibrations in the respective IR-spectra. The hydrogenated graphene forms a greyish highly fluorescent solid. This indicates rather regioselective hydrogenation with the formation of highly functionalized regions next to isolated islands of intact and fluorescent nanographenes. This assumption is corroborated by HRTEM and also observed in related alkylation reactions. Full single-sided hydrogenation of graphene supported on a surface lead to graphone (single-sided graphane). The thermal stability of the system was investigated with temperature programmed X-ray photoelectron spectroscopy (TP-XPS) and desorption (TPD) and, in conjunction with DFT calculations, a complex coverage dependent hydrogenation and dehydrogenation mechanism was discovered.
Both wet-chemical and surface supported approaches have been followed. The highest degree of bulk hydrogenation was obtained in a Birch-type reaction, where frozen water (ice) was used as a gentle proton source. We were able to show, for the first time, that indeed wet chemistry can be used to efficiently generate poly-hydrogenated graphene, which is a milestone achievement on the way to graphane. The reaction product was characterized by statistical Raman spectroscopy and microscopy (SRS, SRM), by IR-spectroscopy, optical spectroscopy (absorption and emission), TGA/MS, and high resolution TEM. For comparison deuterated graphene was also prepared using the same reaction conditions allowing for the unambiguous detection of the expected C-D-vibrations in the respective IR-spectra. The hydrogenated graphene forms a greyish highly fluorescent solid. This indicates rather regioselective hydrogenation with the formation of highly functionalized regions next to isolated islands of intact and fluorescent nanographenes. This assumption is corroborated by HRTEM and also observed in related alkylation reactions. Full single-sided hydrogenation of graphene supported on a surface lead to graphone (single-sided graphane). The thermal stability of the system was investigated with temperature programmed X-ray photoelectron spectroscopy (TP-XPS) and desorption (TPD) and, in conjunction with DFT calculations, a complex coverage dependent hydrogenation and dehydrogenation mechanism was discovered.
 The most complete series of extended [n]cumulenes known to date (n = 3, 4, 7, 9) was synthesized. These molecules offer an in-depth analysis of physical, structural, and electronic properties of model compounds for carbyne a new synthetic carbon allotrope. Theoretical aspects of the electronic properties have been unraveled by quantum mechanical calculations and were related to spectroscopic and electrochemical data. The chemical reactivity of new [n]cumulenes has been explored. Next steps towards the formation of stabilized cumulene derivatives involve the prevention of cycloaddition reactions. A general protocol for polyyne and cumulene rotaxanes has been developed, which offers a new stabilization motif for these molecules; likely through preventing intermolecular dimerization. Among the synthesis and characterization of further endcapped carbon-rods, polyynes equipped with pentacene groups represent a particularly interesting class of compounds. Polyyne-pentacene conjugates have been formed, based on di-, tetra-, and hexa-ynes, and explored theoretically and experimentally as molecular wires. Polyynes turned out to provide an ideal molecular wire with a particularly simple vibronic structure in single-molecule experiments. They allowed understanding the all-important interaction of electron motion with vibrations on a single molecule device.
The most complete series of extended [n]cumulenes known to date (n = 3, 4, 7, 9) was synthesized. These molecules offer an in-depth analysis of physical, structural, and electronic properties of model compounds for carbyne a new synthetic carbon allotrope. Theoretical aspects of the electronic properties have been unraveled by quantum mechanical calculations and were related to spectroscopic and electrochemical data. The chemical reactivity of new [n]cumulenes has been explored. Next steps towards the formation of stabilized cumulene derivatives involve the prevention of cycloaddition reactions. A general protocol for polyyne and cumulene rotaxanes has been developed, which offers a new stabilization motif for these molecules; likely through preventing intermolecular dimerization. Among the synthesis and characterization of further endcapped carbon-rods, polyynes equipped with pentacene groups represent a particularly interesting class of compounds. Polyyne-pentacene conjugates have been formed, based on di-, tetra-, and hexa-ynes, and explored theoretically and experimentally as molecular wires. Polyynes turned out to provide an ideal molecular wire with a particularly simple vibronic structure in single-molecule experiments. They allowed understanding the all-important interaction of electron motion with vibrations on a single molecule device.
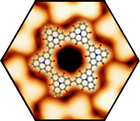 The self-assembly and covalent reaction of heteroatom-containing polycyclic aromatic hydrocarbons (PAHs) on surfaces as well as a molecular level study lead to a series of unprecedented prototypes for two dimensional materials that can be considered as doped and porous graphene and nanoribbon derivatives. Profound understanding of the topographic and electronic structure, stability, and charge transfer effects on metal surfaces using scanning tunneling microscopy was gained. On insulating surfaces it was possible to direct the self-assembly from porous networks to p-p-stacked wire-like aggregates and to determine the structure at the single molecular level by non-contact atomic force microscopy. In addition, the successful production of highly ordered, covalently-linked, two-dimensional porous networks, wires, and macrocycles on coinage metal surfaces through Ullmann-type coupling based on newly developed design rules was accomplished. Towards the formation of non-planar graphyrins from porphyrinoid precursors on surfaces, the reaction of tetraphenylporphyrin yielded the first insight. Also the more complex, and if fully reacted bowl-shaped, Ni(II)-meso-tetrakis (4-tert-butylphenyl) benzoporphyrin (Ni-TTBPBP) was investigated, and the first promising results were found. The reaction of the molecules occurs stepwise: at first only one phenyl group of the Ni-TTBPBP reacts and in a second step the phenyl ring on the opposite side also reacts. Furthermore, graphene grown on Rh(111) was used as a template for the growth of Pd- and Pt-nanoclusters. These clusters are used as model systems for heterogeneous catalysis. In particular, the oxidation of CO was investigated by isothermal XPS measurements, revealing an extremely low activation energy.
The self-assembly and covalent reaction of heteroatom-containing polycyclic aromatic hydrocarbons (PAHs) on surfaces as well as a molecular level study lead to a series of unprecedented prototypes for two dimensional materials that can be considered as doped and porous graphene and nanoribbon derivatives. Profound understanding of the topographic and electronic structure, stability, and charge transfer effects on metal surfaces using scanning tunneling microscopy was gained. On insulating surfaces it was possible to direct the self-assembly from porous networks to p-p-stacked wire-like aggregates and to determine the structure at the single molecular level by non-contact atomic force microscopy. In addition, the successful production of highly ordered, covalently-linked, two-dimensional porous networks, wires, and macrocycles on coinage metal surfaces through Ullmann-type coupling based on newly developed design rules was accomplished. Towards the formation of non-planar graphyrins from porphyrinoid precursors on surfaces, the reaction of tetraphenylporphyrin yielded the first insight. Also the more complex, and if fully reacted bowl-shaped, Ni(II)-meso-tetrakis (4-tert-butylphenyl) benzoporphyrin (Ni-TTBPBP) was investigated, and the first promising results were found. The reaction of the molecules occurs stepwise: at first only one phenyl group of the Ni-TTBPBP reacts and in a second step the phenyl ring on the opposite side also reacts. Furthermore, graphene grown on Rh(111) was used as a template for the growth of Pd- and Pt-nanoclusters. These clusters are used as model systems for heterogeneous catalysis. In particular, the oxidation of CO was investigated by isothermal XPS measurements, revealing an extremely low activation energy.
 Electronic structure calculations revealed that Dirac points not only are present in graphene, but also in various two-dimensional carbon allotropes. In particular graphynes with rectangular structure, which, at first, somewhat unexpectedly, showed that the occurrence of Dirac points is not limited to systems with hexagonal symmetry. A number of potential precursors were synthesized aiming at the formation of new two-dimensional carbon allotropes by chemical vapor deposition on metal surfaces and subsequent self-organized coupling to extended networks. The energetics of coupling reactions on different metal surfaces were determined by DFT calculations and promising combinations of precursors and metal surfaces were identified. Preliminary characterization of these molecular precursors adsorbed onto metal surfaces has been achieved by scanning tunneling microscopy.
Electronic structure calculations revealed that Dirac points not only are present in graphene, but also in various two-dimensional carbon allotropes. In particular graphynes with rectangular structure, which, at first, somewhat unexpectedly, showed that the occurrence of Dirac points is not limited to systems with hexagonal symmetry. A number of potential precursors were synthesized aiming at the formation of new two-dimensional carbon allotropes by chemical vapor deposition on metal surfaces and subsequent self-organized coupling to extended networks. The energetics of coupling reactions on different metal surfaces were determined by DFT calculations and promising combinations of precursors and metal surfaces were identified. Preliminary characterization of these molecular precursors adsorbed onto metal surfaces has been achieved by scanning tunneling microscopy.
 A promising achievement of the first funding period was the establishment of new analytical tools for the efficient and unambiguous structural characterization of SCA derivatives. Whereas “normal” organic molecules – and fullerenes – can be straightforwardly characterized, for example, by NMR spectroscopy, X-ray crystallography, and mass spectrometry, this is not possible for SWCNT or graphene derivatives. The reason is non-volatility, low-solubility and polydispersity. As a consequence, the characterization of reaction products reported in the literature was always rather indirect and unsatisfactorily. To overcome these limitations, we have developed statistical Raman spectroscopy (SRS), statistical Raman microscopy (SRM), and coupled TG/GC/MS analysis. In SRS and SRM, several thousands of spatially resolved Raman spectra of a sample are recorded and quantitatively analyzed by correlation of various observables such as D-, G-, and 2D-band intensities and line shapes. The statistic treatment and representation of these data allows for the quantitative analysis of the degree of functionalization, the sample homogeneity, and the electronic type selectivity of a given reaction. These data (including their temperature dependence) can also be correlated with TG/GC/MS measurements providing exact information about the chemical nature, absolute amount, and cleavage temperature (stability of binding) of covalently and non-covalently bound addends. These new analytical tools are in the meantime being used worldwide for SWCNT and graphene derivative characterization. Analytical ultracentrifugation with a unique multiwavelength detector (MWAUC) was established as a fast method for the size- and shape-analysis of anisotropic carbon allotropes in solution. By applying novel data evaluation methods for MWAUC complex spectral information in the spectral range from 260 to 1050 nm can be extracted together with the hydrodynamic properties of heterogeneous samples. For graphene oxide as a completely exfoliated model compound, a lateral flake diameter distribution was derived from MWAUC data, which is in excellent agreement with the lateral diameter distribution measured traditionally by time consuming atomic force microscopy. Moreover, for completely individualized SWCNTs the hydrodynamic properties and information on the optical properties and chirality could be derived from MWAUC data. Information about the disperse properties of carbon nanodots directly in solution could also be obtained for the first time by MWAUC.
A promising achievement of the first funding period was the establishment of new analytical tools for the efficient and unambiguous structural characterization of SCA derivatives. Whereas “normal” organic molecules – and fullerenes – can be straightforwardly characterized, for example, by NMR spectroscopy, X-ray crystallography, and mass spectrometry, this is not possible for SWCNT or graphene derivatives. The reason is non-volatility, low-solubility and polydispersity. As a consequence, the characterization of reaction products reported in the literature was always rather indirect and unsatisfactorily. To overcome these limitations, we have developed statistical Raman spectroscopy (SRS), statistical Raman microscopy (SRM), and coupled TG/GC/MS analysis. In SRS and SRM, several thousands of spatially resolved Raman spectra of a sample are recorded and quantitatively analyzed by correlation of various observables such as D-, G-, and 2D-band intensities and line shapes. The statistic treatment and representation of these data allows for the quantitative analysis of the degree of functionalization, the sample homogeneity, and the electronic type selectivity of a given reaction. These data (including their temperature dependence) can also be correlated with TG/GC/MS measurements providing exact information about the chemical nature, absolute amount, and cleavage temperature (stability of binding) of covalently and non-covalently bound addends. These new analytical tools are in the meantime being used worldwide for SWCNT and graphene derivative characterization. Analytical ultracentrifugation with a unique multiwavelength detector (MWAUC) was established as a fast method for the size- and shape-analysis of anisotropic carbon allotropes in solution. By applying novel data evaluation methods for MWAUC complex spectral information in the spectral range from 260 to 1050 nm can be extracted together with the hydrodynamic properties of heterogeneous samples. For graphene oxide as a completely exfoliated model compound, a lateral flake diameter distribution was derived from MWAUC data, which is in excellent agreement with the lateral diameter distribution measured traditionally by time consuming atomic force microscopy. Moreover, for completely individualized SWCNTs the hydrodynamic properties and information on the optical properties and chirality could be derived from MWAUC data. Information about the disperse properties of carbon nanodots directly in solution could also be obtained for the first time by MWAUC.
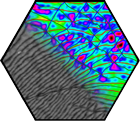 Several groups within the SFB 953 addressed the question of stacked graphene. In particular, the physics of bilayer graphene turned out to be particularly rich due to the nearly unavoidable occurrence of stacking faults: the built-in Bernal stacking provides two equivalent and degenerate domains, typically referred as AB and AC stacking, separated by partial dislocations. Their observation was enabled by the fabrication of robust membranes that lead to the discovery of a dense network of partial dislocations in transmission electron microscopy, with many interesting structural aspects, which were understood in detail by comparison with quantum chemical calculations. Driven by further quantum chemical calculations, we understood that these so-called partials provide very efficient and unavoidable scattering lines in 2D, intersecting the bilayer into a mosaic-like network. In particular, it leads to a linear classical magnetoresistance up to highest accessible magnetic fields and served as a model system. Further, it was found that partial dislocations cause a low-energy blockade of conductance, an effect that was formerly misinterpreted as “insulating phase”. Understanding partial dislocations was the key to solve two long-standing puzzles in solid-state physics. A key requirement for graphene production at technical scale is the understanding of structure – property – process functions. Defect formation was characterized by statistical Raman mapping and related to key processing parameters during graphite delamination.
Several groups within the SFB 953 addressed the question of stacked graphene. In particular, the physics of bilayer graphene turned out to be particularly rich due to the nearly unavoidable occurrence of stacking faults: the built-in Bernal stacking provides two equivalent and degenerate domains, typically referred as AB and AC stacking, separated by partial dislocations. Their observation was enabled by the fabrication of robust membranes that lead to the discovery of a dense network of partial dislocations in transmission electron microscopy, with many interesting structural aspects, which were understood in detail by comparison with quantum chemical calculations. Driven by further quantum chemical calculations, we understood that these so-called partials provide very efficient and unavoidable scattering lines in 2D, intersecting the bilayer into a mosaic-like network. In particular, it leads to a linear classical magnetoresistance up to highest accessible magnetic fields and served as a model system. Further, it was found that partial dislocations cause a low-energy blockade of conductance, an effect that was formerly misinterpreted as “insulating phase”. Understanding partial dislocations was the key to solve two long-standing puzzles in solid-state physics. A key requirement for graphene production at technical scale is the understanding of structure – property – process functions. Defect formation was characterized by statistical Raman mapping and related to key processing parameters during graphite delamination.
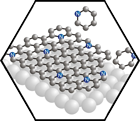 In the first funding period, we gained extensive experience in the preparation of graphene on metal substrates and the chemical modification of graphene with nitrogen and boron leading to heterographene, that is, doped graphene. The formation of these carbon allotropes is achieved via a CVD process using precursors that contain the heteroatom, or by low energy ion implantation. The heterographenes were characterized with photoemission spectroscopy, accompanied by density functional theory calculations. While boron insertion leads to the expected p-doped material, nitrogen insertion results, depending on the functionality in the graphene sheet, in p– or n-doping, for pyridinic or substitutional nitrogen atoms in the graphene lattice, respectively. Extrinsic doping was achieved by means of p-stacking porphyrins and porphycenes onto the basal plane of graphene. In a “molecular” approach, porphyrin derivatives as inherently N-doped carbon-rich species were deposited on surfaces. Stepwise dehydrogenation led to a proof-of-concept that graphyrins or intermediates on the way to graphyrins can be made at specific conditions. Carefully controlled ESI experiments clearly showed the successful synthesis of graphyrins in the gas phase. In parallel to these approaches towards heterographenes, controlled chemical synthesis starting from carefully designed heteroatom-containing polyaromatic precursors (allowing to vary the size, shape, and periphery of the resulting heterographenes), such as polyphenylated porphyrins and bridged triarylamines, were pursued both in solution and on surfaces.
In the first funding period, we gained extensive experience in the preparation of graphene on metal substrates and the chemical modification of graphene with nitrogen and boron leading to heterographene, that is, doped graphene. The formation of these carbon allotropes is achieved via a CVD process using precursors that contain the heteroatom, or by low energy ion implantation. The heterographenes were characterized with photoemission spectroscopy, accompanied by density functional theory calculations. While boron insertion leads to the expected p-doped material, nitrogen insertion results, depending on the functionality in the graphene sheet, in p– or n-doping, for pyridinic or substitutional nitrogen atoms in the graphene lattice, respectively. Extrinsic doping was achieved by means of p-stacking porphyrins and porphycenes onto the basal plane of graphene. In a “molecular” approach, porphyrin derivatives as inherently N-doped carbon-rich species were deposited on surfaces. Stepwise dehydrogenation led to a proof-of-concept that graphyrins or intermediates on the way to graphyrins can be made at specific conditions. Carefully controlled ESI experiments clearly showed the successful synthesis of graphyrins in the gas phase. In parallel to these approaches towards heterographenes, controlled chemical synthesis starting from carefully designed heteroatom-containing polyaromatic precursors (allowing to vary the size, shape, and periphery of the resulting heterographenes), such as polyphenylated porphyrins and bridged triarylamines, were pursued both in solution and on surfaces.
 Charge transport in semiconductor channels of self-assembled monolayers (SAM) functionalized with C60 phosphonic acid derivatives, which are applied in field-effect transistor devices, was investigated using large-scale semiempirical molecular-orbitals calculations and the local ionization energy and electron affinity. The results revealed the specific pathways of electrons through the channel and compare well with Landauer-theory calculations performed for the same system. The simulations of the charge transport pointed to the existence of deep inter-fullerene electron traps, which were subsequently confirmed by cyclic voltammetry and spectro-electrochemistry. Precise knowledge of the charge transport within C60-SAM layers on metal oxides further allows us to understand the electron transport across charge selective interfaces, which is also very relevant for hetero-junction solar cells. Aluminum doped zinc oxide nanoparticles could be successfully decorated with phosphonic acid C60-SAMs and allowed to gain excellent control over the electrical properties of such interfaces by tuning the length of the alkyl spacers as well as the density of the coverage.
Charge transport in semiconductor channels of self-assembled monolayers (SAM) functionalized with C60 phosphonic acid derivatives, which are applied in field-effect transistor devices, was investigated using large-scale semiempirical molecular-orbitals calculations and the local ionization energy and electron affinity. The results revealed the specific pathways of electrons through the channel and compare well with Landauer-theory calculations performed for the same system. The simulations of the charge transport pointed to the existence of deep inter-fullerene electron traps, which were subsequently confirmed by cyclic voltammetry and spectro-electrochemistry. Precise knowledge of the charge transport within C60-SAM layers on metal oxides further allows us to understand the electron transport across charge selective interfaces, which is also very relevant for hetero-junction solar cells. Aluminum doped zinc oxide nanoparticles could be successfully decorated with phosphonic acid C60-SAMs and allowed to gain excellent control over the electrical properties of such interfaces by tuning the length of the alkyl spacers as well as the density of the coverage.
 The enormous synthetic efforts on novel solar-cell materials require a reliable and fast technique for the rapid
screening of novel donor/acceptor combinations in order to
quickly and reliably estimate their optimized parameters. Based on the findings from the detailed balance theory we derived a quantitative Figure of Merit (FoM), which is defined from contactless photoluminescence (PL) measurements and could be shown to precisely anticipate the optimized blend parameters of bulk heterojunction solar cells. Most importantly, this was accomplished without the need of making full devices. A combination of spectrally resolved photoluminescence measurements allows the contactless evaluation of the photoactive layer and can be used to predict the optimized conditions for the best polymer/fullerene combinations. Most interestingly, the contactless, PL-based FOM method has the potential to be integrated as a fast and reliable inline tool for extended material innovation as well as material optimization.
The enormous synthetic efforts on novel solar-cell materials require a reliable and fast technique for the rapid
screening of novel donor/acceptor combinations in order to
quickly and reliably estimate their optimized parameters. Based on the findings from the detailed balance theory we derived a quantitative Figure of Merit (FoM), which is defined from contactless photoluminescence (PL) measurements and could be shown to precisely anticipate the optimized blend parameters of bulk heterojunction solar cells. Most importantly, this was accomplished without the need of making full devices. A combination of spectrally resolved photoluminescence measurements allows the contactless evaluation of the photoactive layer and can be used to predict the optimized conditions for the best polymer/fullerene combinations. Most interestingly, the contactless, PL-based FOM method has the potential to be integrated as a fast and reliable inline tool for extended material innovation as well as material optimization.
 The new developments of SCA chemistry achieved in the first funding period allowed us for the first time to tackle the challenging goal of inter-SCA architecture engineering. In particular, we have shown that active layers of SCAs can be selectively deposited in the channel region of thin film transistor (TFT) devices using a region selective and self-terminating assembly technique. The inherent construction principle relies on a combination of selective covalent-, p‑p‑stacking-, and ion-dipole-interactions. As an example of a 0D system, C60 functionalized with octadecylphosphonic acid addends were used to form self-assembled monolayer field effect transistors (SAMFETs). For non-covalently functionalized SWCNTs and graphene oxide (GO) flakes, electrostatic Coulomb interactions were utilized to implement the selective deposition. Electronically, fullerene-based semiconducting films were contacted by graphene electrodes, where rotational movement of fullerenes plays a key role. In single-molecule experiments using ultra-flat and transparent graphene nanoelectrode pairs and fullerene end-capped molecules, one of the most stunning effects was that the electrical properties are highly insensitive to contact details. Fullerene end-capped molecules and graphene nanoelectrodes provide therefore a material platform that paves the way for a next step in the understanding of charge transport through single molecules, with the potential of linking in operandi optical and electronic spectroscopy, both in stationary and pulsed experiments and facilitating the control of charge transport by desired laser fields. Polyynes and cumulenes as molecular wires complement this inter-SCA toolbox for single-molecule junctions, combining one-dimensionality and a simple vibronic structure.
The new developments of SCA chemistry achieved in the first funding period allowed us for the first time to tackle the challenging goal of inter-SCA architecture engineering. In particular, we have shown that active layers of SCAs can be selectively deposited in the channel region of thin film transistor (TFT) devices using a region selective and self-terminating assembly technique. The inherent construction principle relies on a combination of selective covalent-, p‑p‑stacking-, and ion-dipole-interactions. As an example of a 0D system, C60 functionalized with octadecylphosphonic acid addends were used to form self-assembled monolayer field effect transistors (SAMFETs). For non-covalently functionalized SWCNTs and graphene oxide (GO) flakes, electrostatic Coulomb interactions were utilized to implement the selective deposition. Electronically, fullerene-based semiconducting films were contacted by graphene electrodes, where rotational movement of fullerenes plays a key role. In single-molecule experiments using ultra-flat and transparent graphene nanoelectrode pairs and fullerene end-capped molecules, one of the most stunning effects was that the electrical properties are highly insensitive to contact details. Fullerene end-capped molecules and graphene nanoelectrodes provide therefore a material platform that paves the way for a next step in the understanding of charge transport through single molecules, with the potential of linking in operandi optical and electronic spectroscopy, both in stationary and pulsed experiments and facilitating the control of charge transport by desired laser fields. Polyynes and cumulenes as molecular wires complement this inter-SCA toolbox for single-molecule junctions, combining one-dimensionality and a simple vibronic structure.

