Research
Research topics
Biological Hydrogen Production
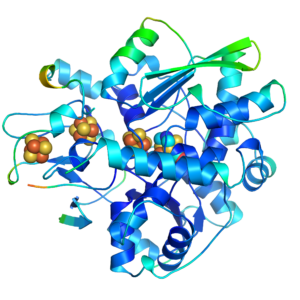
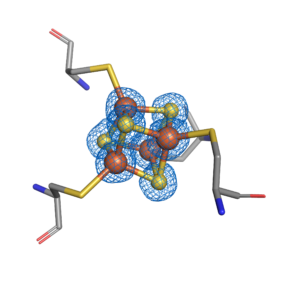
Hydrogen is considered to be an ideal primary energy carrier for a future society based on renewable energy due to its high energy density and clean combustion, which produces only water as a waste product. Hydrogen is envisaged as a significant element of the future fuel mix for transport, enhancing energy security, reducing oil dependency, greenhouse gas emissions and air pollution. Nature has provided us with a series of enzymes that efficiently produce hydrogen with active sites containing only Fe or Ni and Fe, named [FeFe] and [NiFe] hydrogenases. Hydrogenases are bio-catalysts that interconvert protons and electrons and molecular hydrogen in a highly efficient and reversible manner, with rates comparable to that at platinum electrodes, but using the abundant metals nickel and/or iron. While [NiFe] hydrogenases are the most common in nature, it is the [FeFe] hydrogenases that are the most active, but at the same time they are highly oxygen-sensitive. We combine structural, spectroscopic, functional, and theoretical techniques to obtain insight into the active site of the [FeFe] hydrogenases.
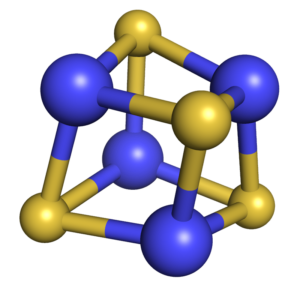
Iron-sulfur Proteins
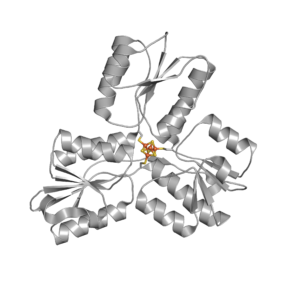
Fe–S clusters are ancient and ubiquitous cofactors in proteins. They are involved in many essential biological processes, including oxidative respiration, photosynthesis, hydrogen production, nitrogen fixation, and DNA replication/repair. The most common species found in proteins are the rhombic [2Fe–2S] and the cubic [4Fe–4S] forms. They are predominantly coordinated by cysteine residues; however, other amino acids can also serve as ligands. The most common role of Fe–S proteins in nature is as electron transfer proteins. Furthermore, the Fe–S cluster also plays important roles in providing stability to protein structures, regulating gene expression, non-redox catalysis, repair and processing of nucleic acids, regulation of cellular processes, and iron homeostasis.
Fighting multiresistant pathogens
The increasing number of infections caused by resistant bacteria is a major thread for public health. Therefore, there is an urgent need for the development of new classes of antibacterial drugs. We are focusing on metalloproteins for a structure-based design of antibacterial drugs.
Understanding the role of Fe-S clusters in important biological processes
Iron–sulfur (Fe–S) clusters are ancient and versatile cofactors that are ubiquitously found in all organisms. Recently, it was discovered that they are involved in the generation of specific high-energy inositol pyrophosphate (IPP) molecules. We are interested in unravelling the role of the Fe-S cluster in the C-terminal pyrophosphatase domain of Schizosaccharomyces pombe Asp1.
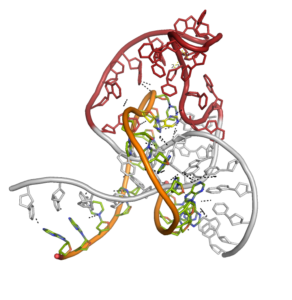
Catalytically active DNA molecules
The unprecedented emergence and spread of SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic, highlights the need for novel strategies for the diagnosis and treatment of viral infections. Single-stranded, catalytically active DNA sequences (DNAzymes) adopt an unusual three-dimensional structure allowing them to catalyse a broad spectrum of reactions. DNA enzymes (DNAzymes) have the potential to provide a platform for the rapid generation of antiviral reagents.
Biological Alkane Oxidation
Alkanes are the most energy-rich form of carbon and are widely spread in the environment. Their transformation by microbes represents a key step in the global carbon cycle. We combine genetic engineering, spectroscopic, and structural methods to unravel the electron transport to the alkane monooxygenase (AlkB), which catalyzes the first step of the microbially-mediated degradation of alkanes.
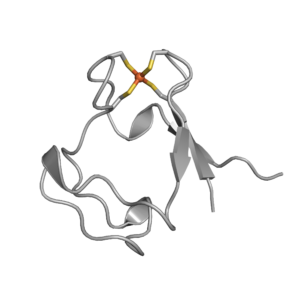 Artificial Metalloenzymes
Artificial Metalloenzymes
The splitting of water into hydrogen and oxygen, ideally using sunlight as the sole source of energy, is considered one of the holy grails of chemistry. Cobalt-based catalysts have emerged as versatile and sustainable compounds for the splitting of water. Our research focuses on generating cobalt-containing biohybrids that can be used for the generation of hydrogen gas.