Research Interests
1. Metal (precursor) complexes via metal evaporization


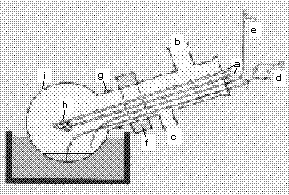
Condensing metal vapors and unsaturated organic compounds including arenes and olefines simultaneously on a cooled wall generally forms very unstable organic substances. Therefore for instance 10 grams of iron can be evaporated within one hour and allowed to react with toluene at -100° C. Although the primary product disintegrates below -60° C, it easily reacts at this temperature forming intermediates which are very useful for a variety of stoichiometric and catalytic reactions. The preparation of metal dotated thermoplastics is also possible using this procedure.
2. Electrochemistry, Electron Paramagnetic Resonance
Intermediates offer redox reactions which result in products of short life. This kind of systems are suitable for electrochemical evidence. Convenient preparative conditions exist if – at room temperature – irreversible electron transfer reactions are obtained, which change to a reversible behaviour in the cold. These intermediates, which may be short-lived or stable only at lower temperatures, usually exhibit an odd number of electrons and so allow the use of EPR (Electron Paramagnetic Resonance). These products can also be obtained by in situ reduction on the surface of an electrode. Other applications of EPR in combination with electrochemical techniques are also used in various areas of chemistry.
